Silicone,
and Acrylic IOLs
on
Retinal Image Contrast
and
Functional Visual Performance
Robert
M. Kershner, MD, FACS
Eye
Laser Center
Suite
303, 1925 West Orange Grove Road
Tucson,
Arizona 85704-1152 USA
Phone:
(520) 797-2020 Fax:
(520) 797-2235
e-Mail:
Kershner@EyeLaserCenter.com
©2003. Robert M. Kershner, M.D., F.A.C.S. All rights reserved.
No
portion of this paper can be copied, reproduced in any way or distributed
without the expressed written permission of the copyright holder.
This prospective, randomized study compares an aspheric IOL with conventional spherical silicone and acrylic lenses on retinal image contrast and functional visual performance. 221 eyes of 156 patients were randomly assigned to receive one of each of the three intraocular lenses with six months of follow-up. Measured parameters include visual acuity, fundus photographic retinal image contrast, and functional acuity contrast testing. The differences in the pre and postoperative spherical and astigmatic refractive error and preoperative best-corrected visual acuity between groups are not statistically significant. In the first postoperative month uncorrected visual acuity is best in the aspheric group. The aspheric IOL group exhibit up to a 47% increase in contrast for photopic, 38% in photopic with glare, 100% in mesopic and 100% in mesopic with glare functional acuity contrast testing. Acrylic IOLs show no increase in photopic, up to 38% increase in photopic with glare, 50% in mesopic and 50% in mesopic with glare. Spherical silicone IOLs show no increase in contrast testing when compared to cataract. Digital analysis of retinal imaging demonstrate increased threshold luminance levels in the aspheric group (range 116-208) and a four-fold increase in image contrast compared to the silicone and acrylic groups. The aspheric IOL (Tecnis) provides significant improvement in objective retinal image contrast and in visual performance as measured by visual acuity and functional acuity contrast testing. This improvement was most pronounced in night vision and night vision with glare contrast testing when compared with conventional spherical silicone and acrylic IOLs.
Abstract
:
A
Prospective Evaluation of Aspheric (Tecnis), Silicone and Acrylic IOLs
on Retinal Image Contrast and Functional Visual Performance
Robert
M. Kershner, M.D., F.A.C.S., A.B.E.S.
Purpose: Studies have shown that the loss of balance between the positive
spherical aberration of the cornea and the crystalline lens seen with
increasing age may be one cause of decreased contrast sensitivity in
cataract patients. Conventional spherical intraocular lens (IOL) optics
correct spherical error only and may add to the positive spherical
aberration. The recent introduction of an anterior modified prolate,
aspheric intraocular lens (Tecnis) may correct this aberration and
result in an increase in contrast sensitivity in post cataract surgery
patients. This study was designed to compare an aspheric IOL with
conventional silicone and acrylic lenses on retinal imaging and
functional visual performance.
Setting: Eye Laser Center, Tucson, Arizona USA
Methods: 221 eyes of 156 patients were randomly assigned to
receive one of each of the three intraocular lenses. Visual acuity was
measured preoperatively, at one day, one week, three weeks, one month,
three months and six months postoperatively. Fundus photography and
photopic and mesopic functional acuity contrast testing was performed
preoperatively and at three months postoperatively.
Results: The differences in the pre and postoperative spherical and
astigmatic refractive error and best-corrected visual acuity between
groups were not statistically significant. Postoperative uncorrected
visual acuity was best in the aspheric group in the first month. The
aspheric IOL group had a 38-47% increase in photopic, 38% in photopic
with glare, 43-100% increase in mesopic and 9-100% increase in mesopic
with glare, functional acuity contrast testing. Acrylic IOLs showed no
increase in photopic, 38% increase in photopic with glare, 25-50%
increase in mesopic and 36-50% increase in mesopic with glare functional
acuity contrast testing. Spherical silicone IOLs showed no increase in
contrast testing when compared to cataract. Digital analysis of retinal
imaging demonstrated increased threshold luminance levels in the
aspheric group (range 116-208) and a four-fold increase in image
contrast compared to preoperative cataract, silicone and acrylic groups.
Conclusions: All three intraocular lenses demonstrate improved visual
acuity following cataract surgery. The aspheric IOL (Tecnis) provides
significant improvement in retinal image contrast and in visual
performance as measured by visual acuity and functional acuity contrast
testing. This improved visual performance was greatest in the testing of
night vision and night vision with glare when compared with conventional
spherical silicone and acrylic IOLs.
Introduction
|
The
invention of the intraocular lens (IOL) by the late Sir Harold Ridley of
England in 1949 heralded a new era in the surgical treatment of cataract.
Implantation of IOLs eliminated the need for bulky, aphakic spectacles and
provided cataract patients with near normal vision. Small
corneal incisions, topical anesthesia and the use of microinstrumentation
in cataract surgery has further advanced the science of providing clear,
uncorrected vision for millions of patients who undergo cataract surgery1.
The rigid glass and plastic IOLs of Ridley's era have yielded to newer
foldable and injectable IOLs. Small incision surgery2,3 has
made it possible to reduce incision size to less than 3.0 mm
(microincision). The preexisting spherical myopia, hyperopia and even
astigmatism can be corrected with judicious selection of an injectable IOL4.
The
estimated 1,498 existing IOLs in present use from 33 different
manufacturers5 all contain a convex anterior refracting surface
that approximates a sphere. This past year, the United States Food and
Drug Administration approved for use in the United States an anterior,
modified prolate, aspheric IOL design that has an optic that is steeper in
the center and flatter in the periphery (Tecnis Z-9000™,
Pharmacia Corporation, Peapack, NJ USA). The theory behind creating such an IOL is based upon
observations that a positive spherical aberration of the cornea is
balanced by the negative spherical aberration of the crystalline lens in
youth. The increasing positive spherical aberration of the crystalline
lens in the aging eye fails to neutralize this aberration and contrast
sensitivity decreases6. Spherical IOLs do not correct for this
aberration and in fact may compound the error7. Objective
assessment of retinal imaging suggests that the retinal images of
postcataract surgery patients are similar or worse than that of healthy
patients of the same age7-8. Evaluating postcataract patients
with conventional measurements of visual acuity using high contrast
letters on a white background, as is tested with the Snellen chart first
used in 1865, measure only within a narrow range of visual function.
Measuring contrast sensitivity under different lighting conditions may
allow a better evaluation of a patient with 20/20 vision following
cataract surgery who is still visually disabled at night. Combined,
Snellen acuity and functional acuity contrast vision testing may be a more
sensitive indicator of true visual performance. Digitally analyzing fundus
photographic retinal images obtained from cataract patients before and
after IOL implantation, can be applied in an innovative way to measure the
differences in image contrast between the three lenses. This study was
undertaken to assess visual acuity and contrast sensitivity, in analyzing
the visual performance of three different IOLs in post cataract patients
under different lighting conditions. The goal of this study is to compare
the difference in IOLs based upon lens material and surface optics to
determine if the addition of an aspheric optic can improve retinal image
contrast, visual and contrast acuity, and therefore visual performance. |
Materials
and Methods
The
study was designed as a six month, prospective, randomized evaluation of three
commonly used though different IOLs. Informed consent, which met the standard
for the Institutional Review Board where the study was performed, was obtained
from all patients enrolled. Each
patient received a comprehensive ophthalmic examination which included tonometry,
refraction, slit-lamp and dilated fundus examination. Patients with preexisting
glaucoma, diabetes or macular pathology were excluded from the study.
Whenever possible, if the patient required cataract surgery on both eyes,
they received the same IOL for the fellow eye. Data was collected from each eye,
entered onto a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond,
Washington, USA) for each group and analyzed following the conclusion of the
study. A total of 221 eyes from 156 patients were enrolled over three months and
each followed for a total of six months. The average age was 71.6 years, and the
ratio of females to males was 1.5:1. There was no loss of patient data due to
attrition during the course of the study. Each patient was randomized to one of
three groups for the lens to be implanted, a one-piece silicone, one-piece
acrylic, or three-piece aspheric (Tecnis) IOL. Surgery was performed by a single
surgeon experienced in microincision cataract surgery, (RMK), utilizing the same
surgical methods for each lens implantation. Best corrected preoperative visual
acuity, and uncorrected postoperative acuity was obtained in a controlled
testing environment using a projected Snellen chart, calibrated illumination and
room lighting and was measured by the same certified ophthalmic technician. One
certified ophthalmic technician was assigned to obtain pre and postoperative
functional contrast acuity testing, and one was assigned to obtain pre and
postoperative fundus photography for all patients. All data was collected with
the observers blinded as to type of IOL being tested. Patient's pupils were
measured to conform to a range of 3-5mm without dilation for photography. Fundus
photographs were obtained with a Canon CR4-45NM Dual non-mydriatic retinal
camera preoperatively, at three months postoperatively, and analyzed using Adobe
Photoshop 7.0 image software (Adobe Systems Incorporated, San Jose, California,
USA) using the L*a*b color model 9.
The L*a*b color model is based on the model proposed by the Commission
Internationale d'Eclairage (CIE) in 1931 as an international standard for color
measurement. In 1976, this model was refined and named CIE L*a*b.
L*a*b color is designed to be device independent, creating consistent
color regardless of the device (such as a monitor, printer, computer, digital
camera or scanner) used to create or output the image. L*a*b color consists of a
luminance or lightness component (L) and two chromatic components: the ‘a’
component (from green to red) and the ‘b’ component (from blue to
yellow). This software formula was applied to analyze the fundus photographs for
luminance and contrast and display the data as a surface area chart where the
‘x’ axis is luminance, displayed over the ‘y’ axis which is the color
spectrum. In Photoshop, the L*a*b mode has a lightness component (L) that can
range from 0 to 100. The ‘a’ component (green-red axis) and the b component
(blue-yellow axis) can range from +120 to -120. The threshold luminosity
histogram can be displayed in numeric values from 0 to 250 representing the
difference from white (low values) to black (high values). The digital data is
represented numerically as the mean ± standard deviation (SD) tabulated for
each group.
All
patients received preoperative computed topography (Tomey Corporation, Computed
Anatomy Tomography), and ultrasonic A-scan biometry with a Sonomed A-1500 A-Scan
unit (Sonomed, Lake Success, NY USA). Keratometry was performed with a Bausch and Lomb
keratometer (Bausch and Lomb, Rochester, NY).
Calculations for IOL power selected for implantation were based
upon a predicted of final correction of -0.25 to –0.50 D. The pooled data were
applied from the average calculations obtained from the Binkhorst I, SRK II and
Holladay IOL formulas.
Contrast
Testing
Contrast
refers to the difference in brightness levels from one part of an image to
another. The sine-wave grating
method of testing is based upon the presentation of a basic target pattern of
fuzzy gray bars. To provide a maximum of sensitivity, the bars are spaced closer
together as higher contrast is tested. The image size can then be measured as a
spatial frequency, the higher the number, the smaller the image size. Scotopic
testing refers to levels of illumination less than 0.05 lux, (where essentially
only rods are functioning and therefore is not tested in this study), mesopic
testing between 0.05 lux and 50 lux and photopic testing greater than 50 lux10.
The graphic representation of contrast sensitivity is recorded as a log function
on the y axis and displayed for each of the five spatial frequency targets on
the x axis (1.5, 3.0, 6.0 12.0 and 18.0 cycles per degree (CPD). A single log
unit increase therefore would represent a ten-fold increase in contrast
sensitivity.
Functional
acuity contrast testing (F.A.C.T.) as developed by Arthur P. Ginsburg, Ph.D.11
was performed with best spectacle correction for the target distance using a
Stereo Optical Optec 3500 Vision Testing System (Stereo Optical Company,
Chicago, IL USA), with sine-wave grating charts at levels of illumination for
day (photopic) at 10 lux with a target illumination value of 85cd/m2, and night (mesopic) at 1 lux with a target illumination
value of 3 cd/m2. Glare luminance
levels were set at 1 lux for night glare and at 10 lux for day glare.
The mean (± SD) of the log contrast sensitivity values are displayed in
the graphs. The log base 10 of these values were used to plot the contrast
sensitivity on the 'y' axis for each spatial threshold. The two-tailed t-test
was used for comparing each group value. A difference of 0.15 log units is
considered statistically significant.
The
IOLs. 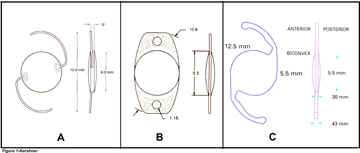
Figure
1-Diagrams of A) Aspheric TECNIS Z-9000, B) Silicone
STAAR AA4207VF and C) Acrylic ACRYSOF SA60AT IOLS.
One-piece silicone-105
eyes of 64 patients received the STAAR elastic AA4207-VF Silicone IOL, a 10.8mm
overall length plate haptic IOL with 00
haptic angulation and a 5.5 mm biconvex 1:1 ratio of the curvature of the optic
(figure 1A). It is manufactured from an elastic RMX3-UV, ultraviolet absorbing
silicone with an index of refraction of 1.413 and is designed to be implanted
completely within the capsular bag following phacoemulsification cataract
extraction. The IOL can be folded for insertion through an incision size of
3.5mm or smaller and is available in powers of +4.0 to +34.0 diopters.
(STAAR Surgical Company,
Monrovia, California, USA).
One-piece
acrylic-41 eyes of 27 patients received the ALCON SA60AT acrylic IOL, a 13.0
mm single piece overall length IOL with a 6.0 mm diameter, anterior asymmetric,
biconvex optic, with 00
haptic
angulation (figure 1B).
It is manufactured from an ultraviolet-absorbing acrylate/methacrylate
copolymer with an index of refraction of 1.55 in available powers of +6.0 to
+34.0 diopters. The material is capable of being folded prior to insertion and
is intended to be inserted into the capsular bag following cataract removal (ALCON
Laboratories, Inc. Ft. Worth, Texas, USA).
Three-Piece
Silicone, Aspheric-
75 eyes of 65 patients received the Pharmacia Tecnis Z-9000 Silicone IOL, a
12.0mm overall diameter, three-piece equibiconvex, 6.0mm optic with square edges
and a 60 haptic angulation (figure 1C). It is manufactured from an
elastic, ultraviolet absorbing polysiloxane with a refractive index of 1.46. The
anterior lens surface has a modified prolate, aspheric design which is thicker
in the center and thinner in the periphery to minimize spherical aberration. The
two haptics are configured into a capsular 'C' shape and are manufactured out of
polyvinylidene fluoride (PVDF). The lens is designed to be implanted completely
within the capsular bag following phacoemulsification cataract extraction. It is
capable of being folded for insertion through an incision size of 3.5mm or
smaller and is available in powers of +5.0 to +34.0 diopters.
(Pharmacia Corporation, Peapack, New Jersey USA).
Surgical procedure
The
operative eye was prepared for surgery with 1 drop of 1% cyclopentolate
hydrochloride, and 2.5% phenylephrine hydrochloride (Cyclomydril, Alcon, Ft.
Worth, Texas USA) starting 15 minutes prior to surgery with drops repeated once
every five minutes for a total of three drops. Topical povidone iodine solution (Betadine 5%, Escalon, Lakewood, NJ USA) was instilled 10 minutes prior to
surgery into the operative eye. The technique of topical anesthesia has been
previously published.12-15 Topical 0.5% tetracaine HCL (CibaVision,
Atlanta, GA USA) was instilled at the start of the prep and before the procedure
commenced. The eye and eyelid skin were prepared with Betadine solution and
draped. A 2.85 mm trifaceted diamond keratome (Kershner Corneotome-Diamatrix,
The Woodlands, Texas USA) for the silicone and aspheric IOLs or a 3.2 mm Becton
Dickinson Clear Cornea Incision System slit blade (BD Ophthalmic Systems,
Waltham, MA USA) for the acrylic IOLs, was used to create a clear cornea
incision, just anterior to the limbus entering the anterior chamber parallel to
the iris plane. There was no paracentesis and no attempt was made to correct
preexisiting astigmatism with additional incisions for this study. The
techniques of capsulorrhexis with the Kershner one-step capsulorrhexis forceps
(Rhein Medical, Tampa, FL USA)16,17 hydrodissection,18
single-incision, single-instrument intercapsular phacoemulsification,19
and intercapsular implantation of the injectable intraocular lens,20,21
were then followed in order. Viscoelastic was used for the capsulorrhexis and
filling of the capsular bag for IOL implantation (Healon, Healon GV and
Healon-5, Pharmacia, Inc. Peapack, New Jersey USA). Phacoemulsification was
performed with the Bausch and Lomb Millennium phacoemulsification unit (Bausch
and Lomb Surgical, Rochester, NY USA) using linear surgeon control with a 30
degree phacoemulsification tip and custom-fitting teflon sleeve at maximum phaco
powers of 20% - 30%. The lens bag was inflated with viscoelastic. A STAAR MSI-TR
injector with disposable ST-45 cartridge for the STAAR silicone lens, the ALCON
Monarch II injector with disposable Monarch cartridge for the acrylic IOL, and
the Kershner-Mastel MicroInjector with disposable cartridge (Mastel Precision,
Rapid City, South Dakota USA) for the aspheric IOL, was used to fold and insert
the IOL through the unenlarged clear cornea incision into the capsular bag. All
viscoelastic was removed with irrigation and aspiration. The incision was
self-sealing and did not require hydration. No intracameral antibiotics or
miotics were used. The total operative time for the procedure averaged four to
six minutes. The eye was not patched at the conclusion of the procedure and the
patient was allowed unrestricted activities following surgery.
Postoperative Procedure
Patients were started on topical tobramycin-dexamethasone solution (Tobradex-Alcon, Ft. Worth, Texas USA) one drop into the operative eye four times a day commencing the day of the procedure, and continuing for ten days. They were allowed to use artificial tear supplements as needed. No other postoperative drops or medications were used. Patients were examined on the first postoperative day, at one-week, three weeks, one month, three months and six months following their surgery. All patients underwent measurement of uncorrected visual acuity, a full slit lamp examination, refraction, best-corrected acuity and applanation tonometry on each postoperative visit. Patients were provided with spectacle correction in the form of a reading glass or bifocal with their full refraction at the one-month postoperative visit. Funduscopic photography and contrast acuity testing with best correction was obtained at the three month postoperative examination during the study period.
Results
There
were no surgical or postoperative complications observed during the course of
this study. Thirty-one patients (7%), unassociated with lens style, had
postoperative pressure rises exceeding 24mm Hg on the first postoperative day
that were relieved with releasing aqueous and the application of one drop of
0.5% Timolol (Merck and Co., Inc.). The YAG capsulotomy rate for the group at
six months was 0.9% for the silicone, and 0% for the aspheric and acrylic IOLs.
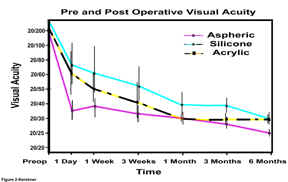
Figure
2-Preoperative best-corrected and postoperative
uncorrected visual acuity at six months for each of the IOL groups
The preoperative best-corrected visual acuity of 20/265±23 for the three groups {range 20/50-counting fingers} was not statistically significant between groups (Figure 2). Postoperatively, the best corrected visual acuity at one week for each of the three IOL groups, silicone 20/40±8, acrylic 20/40±11, and aspheric 20/38±5 were also not statistically significant. However, the data for uncorrected postoperative visual acuity (figure 2) is statistically significant. On postoperative day 1, the acuities for the silicone group were the poorest at 20/73±8, better with the acrylic group 20/59±11, with the best results seen in the aspheric group at 20/35±3. This trend was maintained until the one-month visit, when the acrylic IOLs matched the uncorrected acuity of the aspheric. There was a statistically significant difference in uncorrected acuity that continued from the three to six month visit between the aspheric group (20/25±2) and the silicone (20/36±1) and acrylic (20/30±1) groups at six months. This difference could not be explained on the basis of preexisting or postoperative refractive error as can be seen in figure 3.
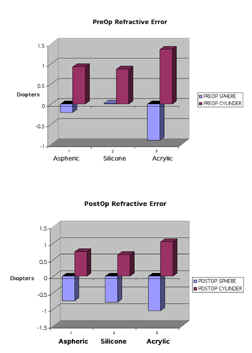
Figure
3-A) Preoperative and B) postoperative spherical and
astigmatic refractive error for each group
Preoperatively, the
acrylic group had an average refractive error of [–0.9(±1.02)+1.36(±0.78)
diopters [D]], the aspheric (-0.21(±1.24) +0.93(±0.45) D) and the silicone
(-0.02 (±0.03) +0.87(±0.32) D). The preoperative spherical equivalents for
each group were (-0.22 acrylic, -0.26 silicone, +0.46 aspheric).
Postoperatively, the acrylic group had an average refractive error of (–1.05(±1.09)+1.04(±0.58)
D), the silicone (-0.79 (±0.57)+0.65(±0.3) D) and the aspheric (-0.75(±0.78)+0.74(±0.43)
D). The postoperative spherical equivalents for each group were not
statistically significant (-0.53
acrylic, -0.47 silicone, -0.38 aspheric).
Figure 4 displays the mean (±SD) of the functional acuity contrast testing for each of the four groups under four different levels of luminance: A) daylight (photopic), B) daylight with glare, C) night (mesopic), and D) night with glare, for each of the five spatial frequencies tested.
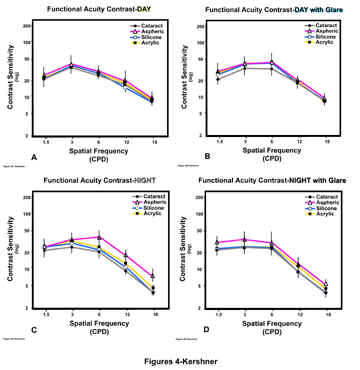
Figure
4-A) Comparison of aspheric, silicone, acrylic IOLs and
preoperative cataract on Functional Acuity Contrast Testing (F.A.C.T.) of day
vision (Photopic)
B) Comparison of aspheric, silicone and acrylic IOLs
on Functional Acuity Contrast Testing (F.A.C.T.) of day vision with glare
C) Comparison of aspheric, silicone, acrylic IOLs
and preoperative cataract on Functional Acuity Contrast Testing (F.A.C.T.) of
night vision (Mesopic)
D) Comparison of aspheric, silicone, acrylic IOLs
and preoperative cataract on Functional Acuity Contrast Testing (F.A.C.T.) of
night vision with glare
In
figure 4A are displayed the four curves for the contrast testing of patient’s
individualeyes under daylight lighting (photopic). As would be expected based
upon published data7,the contrast sensitivity of cataract patients
is the lowest throughout all the spatial frequencies tested. There is
improvement in the mean functional contrast acuity under photopic testing
conditions, with each of the IOLs implanted. The differences between the
preoperative cataract and the silicone and acrylic IOLs were not statistically
significant. The aspheric IOL demonstrated increased contrast sensitivity for
each spatial frequency tested under photopic conditions. The values for the
aspheric group displayed in figure 4A represent statistically significant
improved contrast testing of 38% at spatial frequencies of 1.5 to 6 cpd, and 47%
at 12 cpd.
In
figure 4B are displayed the four curves for the mean values (±SD) for contrast
testing of patient’s individual eyes in daylight (photopic) lighting with
glare. The levels for the preoperative cataract are the lowest. All three IOL
groups show a 38% improvement in contrast over preoperative values at spatial
frequencies of 1.5, 3, and 6 cpd. There was no statistically significant
difference within the three IOL groups for photopic contrast testing with glare.
The
differences between preoperative cataract and the postoperative IOL groups
become most apparent with the data displayed in figure 4C for functional acuity
contrast testing in dark (mesopic) conditions. Again, preoperative cataract
demonstrates the lowest levels of contrast sensitivity tested. There is no
statistically significant difference in contrast values between any of the
groups at spatial frequencies of 1.5 or 3.0 cpd. Silicone IOLs show no
improvement over the cataract group at any spatial frequency. At 6.0 cpd, the
differences become significant. Acrylic IOLs show improved contrast values of
26% over the cataract, the aspheric IOL group demonstrates improved contrast of
43%. At 12 cpd, the acrylic shows a
25% improvement over the cataract group, the aspheric 63%. At 18 cpd, or the
smallest target patch presented to the patients, the acrylic IOL group had a 50%
improvement in their contrast sensitivity over the preoperative levels, the
aspheric IOL group had a 100%.
In
figure 4D, the difference in contrast values for night (mesopic) levels with
glare testing at 1.5, 3, 6 cpd, for the silicone, acrylic and preoperative
cataract groups were not statistically significant. Silicone IOLs show no
improvement over the cataract group at any spatial frequency. Aspheric IOLs show
increased contrast of 9% over cataract at 1.5 cpd, 23% at 3 and 6 cpd, 43% at 12 cpd, and 100% at 18
cpd. Contrast testing in the acrylic IOL group was improved
36% over cataract at 12 cpd, and 50% at 18 cpd.
The fundus photographs from each group were individually analyzed for contrast using the Adobe Photoshop program creating luminosity histograms for each image and then analyzing the maps for the thresholds from the lightest to the darkest for each image (contrast). This software analysis is displayed as a threshold luminance map. Three representative sets of photographs are shown in figure 5.
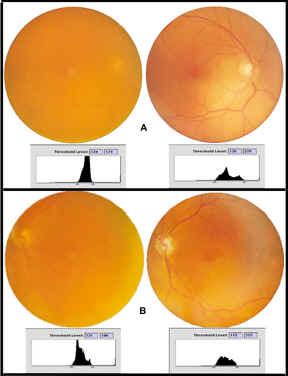
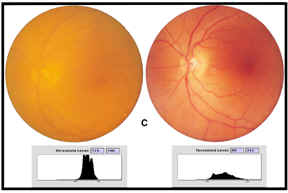
Figure
5-A) Fundus photography of preoperative cataract (left image) and postoperative
silicone IOL (right image) at 3 months with threshold luminance maps
B)
Fundus photography of Preoperative Cataract (left image) and postoperative
acrylic IOL (right image) at 3 months with threshold luminance maps
C)
Fundus photography of preoperative cataract (left image) and postoperative
aspheric IOL (right image) at 3 months with threshold luminance maps
In set (A) the
preoperative fundus photograph of an eye as imaged through the cataract is
displayed on the left, the postoperative fundus photograph of the same eye as
imaged through the AA4207VF Silicone IOL is displayed on the right.
Beneath each photograph is the threshold luminance map for that image. In
set (B) the preoperative fundus photograph as imaged through the cataract of an
eye is displayed on the left, the postoperative fundus photograph as imaged
through the same eye through the SA60AT Acrylic IOL is displayed on the right.
In set (C) the preoperative fundus photograph as imaged through the cataract of
an eye is displayed on the left, the postoperative fundus photograph as imaged
through the aspheric Tecnis Z-9000 IOL of the same eye is displayed on the
right. When comparing the images on the left to those on the right, the clarity
and contrast of all three images are markedly improved following removal of the
cataract and implantation of an IOL.
Contrast can be mathematically defined as the difference between the brightness of the light edge (low threshold luminance [L]), subtracted from the dark edge (highest threshold luminance), divided by the sum of the two brightnesses (c=(Lmax-Lmin)/(Lmax+Lmin). This numerical value is displayed in decimal form as a percent of contrast. In each case, the threshold luminance map demonstrates that the image through the cataract contains the least difference between light and dark, or in other words, the least contrast to the image (A=124 to 174 threshold range of 50, contrast level 0.17), (B=121 to 186 threshold range of 65, contrast level 0.21), (C=115 to 190 threshold range of 75, contrast level 0.25). The histogram for each fundus image through the cataract shows tall and narrow peaks representing all the color data for that image crowded between the lightest (on the left of the histogram) to the darkest (on the right of the histogram). When compared to the postoperative fundus photographs for each group on the right, there is a marked improvement in contrast for each IOL from the baseline cataract image (A=120 to 229 threshold range of 109, contrast level 0.31, B=115 to 215 threshold range of 100, contrast of 0.30, C=89 to 212 threshold range of 127, contrast level 0.42). Postoperative fundus images show short and broad peaks over a larger area representing much more color data separating the lightest to the darkest. These flatter and broader luminance maps compare (A) silicone, AA4207VF to (B) acrylic, SA60AT to (C) aspheric Tecnis Z-9000 IOLs shows the greatest contrast over the broadest color range from red to green to blue (RGB). The greater the difference within each threshold luminance map, the greater the contrast in the image taken through the IOL. The mean contrast threshold is 109 for the silicone AA4207VF and 127 for the aspheric (Tecnis) IOL. The lowest mean contrast threshold is for the acrylic SA60AT (100). The lowest threshold luminance for light is 89 for the aspheric Tecnis Z-9000 photograph. This represents the lowest light level imaging of the fundus. The image taken through the aspheric IOL demonstrates the greatest contrast level (percent contrast) at 0.42. The photographic data represented in figure 5 is corroborated by the pooled numerical data for the threshold luminance maps from all the fundus images obtained at three months for each group following surgery (figure 6).
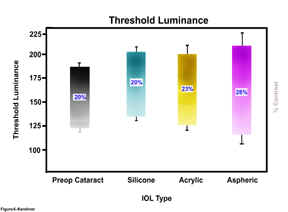
Figure
6-Threshold
Luminance Ranges for preoperative cataract, silicone, acrylic and aspheric IOLs (with percent
contrast for each group)
The mean range in threshold luminance for the preoperative cataract
retinal images (contrast) from the lightest levels is 120±5 to the darkest
levels of 183±8 (difference=63, contrast level 0.20). Analysis of the
postoperative imaging demonstrates an improvement in range for the silicone
group from lightest of 134±5 to darkest of 203±8 (difference=69, contrast
level 0.20), for the acrylic group from lightest of 123±7 to darkest of 195±17
(difference=72, contrast level 0.23), and for the aspheric group from lightest
of 116±13 to darkest of 207±10 (difference=91, contrast level 0.28). The
aspheric IOL group demonstrated the greatest statistically significant
difference in the range of threshold luminance from lowest to highest. The
aspheric IOL group also demonstrated the greatest contrast level of the images
of 0.28. As the threshold luminance graph illustrates, the lowest levels of the
image contrast for the silicone and acrylic groups is greater than that for the
cataract itself, demonstrating that the contrast in the low light range is
poorer for the images taken through these IOLs as a group, than in the
preoperative cataract group. By contrast, the aspheric group imaged the best in
the low light contrast areas with the lowest threshold luminance of all four
groups. At the highest threshold
luminance levels, the aspheric IOL group measured higher than the cataract, the
silicone and the acrylic groups. These differences are statistically significant
(P<0.001).
The
contrast levels of the images obtained through the silicone IOLs (0.20) is the
same as through the cataract (0.20). The
contrast level of the images obtained through the acrylic IOLs is (0.23).
Comparatively, the contrast levels of the images obtained through the aspheric
IOLs (0.28) represents a 40% increase in contrast of the postoperative images.
These differences are significant (P<0.001).
Discussion
For
over one hundred and thirty-eight years, ophthalmologists have been measuring
visual acuity using a high contrast Snellen visual acuity chart.
Unfortunately, this is but one measure of a patient's true visual
performance. Individuals are living longer, more independent lives, and need to
meet the demands of daily living with better visual acuity. The technology and
benefits of modern cataract surgery are accelerating at a tremendous pace,
resulting in better visual outcomes and better uncorrected visual acuity for our
patients1. This places greater demands upon ophthalmic surgeons to
deliver near-normal vision. Patients require more than just better acuity, they
need better functional visual performance under all lighting conditions. The
ability to compare and differentiate the differences between light and dark,
especially under low light (mesopic) conditions is critical to such tasks as
driving and reading signage. Combining the measurement of functional acuity
contrast testing with visual acuity testing adds important information to our
knowledge of visual performance. The potential visual disability that may come
with reduced contrast sensitivity can interfere with a patient's ability to
drive, especially at night. Owsley et al. performed a cross-sectional analysis
on 274 older drivers and found that driver's with a history of crash involvement
were eight times more likely to have a serious contrast sensitivity deficit in
the worse eye than those who were crash free22. Contrast sensitivity
decreases with advancing age23-24.
It would be expected then that cataract patients would be most likely to
be affected by a decrease in contrast sensitivity.
This
study demonstrates that modern clear cornea cataract surgical techniques, with
the implantation of a flexible and foldable IOL, results in a low incidence of
complications and excellent visual outcomes. What this study set out to
determine is if there is a difference in visual performance between conventional
spherical optics of the flexible and foldable silicone and acrylic IOLs and the
anterior surface, modified prolate, aspheric IOL. This study did not control for
the type of lens material or the style of the implant, as each lens in the study
was intentionally different. In a limited preliminary study, I compared the 911A
a 6.0mm optic, three-piece IOL with PVDF haptics (Pharmacia Corporation, Peapack,
NJ) with the Tecnis Z-9000 aspheric IOL (unpublished results-Kershner). The 911A
is an identical style IOL with the same material, haptic design and shape. It
did not demonstrate the added improvement in contrast acuity testing that was
demonstrated by the identical lens (Tecnis) with the aspheric optic in this
study. This data was also confirmed by a study by Holladay et al. in
which they compared a spherically surfaced equi-biconvex lens of the same power
with the anterior prolate IOL. Their results in improved contrast from the
optical properties of the prolate lens were not shared by the same lens and
design without the prolate surface25.
It
is difficult to postulate that haptic design or lens style would contribute to a
difference in contrast sensitivity delivered through the optic and therefore no
attempt was made to control these factors in this study. A study comparing the
aspheric IOL optic with two commonly implanted though different lens materials
and styles is but one approach to determine if the addition of an aspheric optic
surface would impact contrast sensitivity and image contrast.
This
study did not control for pupil size. It has been demonstrated that the
anterior, modified prolate, aspheric optic of the Tecnis Z-9000 IOL demonstrates
superior optical performance when compared to spherical IOLs for systems with
5-6mm pupils. It is postulated that the larger the pupil, the greater the
contribution from the degrading effects of optical aberrations25.
Therefore, one would expect the optical performance of this aspheric IOL
in this study to be superior under conditions where pupil size would be larger (mesopic
and mesopic with glare testing). The data in this study confirms this
hypothesis. It has also been shown with optical bench testing, that lens
centration of less than 0.4mm and lens tilt of less than 7 degrees maximize the
effects of the aspheric IOL25. This
study made no effort to determine lens tilt or centration of any of the IOLs.
With today’s advanced microincision cataract surgery techniques, it may be
expected that these parameters are well within the tolerances for IOL
implantation26.
A
review of the data on visual acuity, shows that uncorrected visual acuity in the
first four weeks after surgery was better in the aspheric group when compared to
silicone and acrylic IOLs (figure 2). The uncorrected visual acuities in the
acrylic IOL group were better than the silicone but did not improve to the level
of the aspheric IOL group until one month postoperatively. At six months there
still was a difference in uncorrected acuity with the aspheric IOL group
achieving the best visual levels. This difference cannot be explained on the
basis of refractive error alone. The difference in spherical equivalent was not
statistically significant following surgery between the groups. The differences
may be attributed in part to the size of the incision, the acrylic IOL requiring
a slightly larger incision than the silicone. However, the one-piece silicone
IOL had uncorrected visual acuity that was not as good as the acrylic or
aspheric IOLs during the course of this study and the incision sizes were
essentially identical to the aspheric IOL group. There may have been slightly
greater astigmatism or corneal edema in the postoperative course for these eyes,
however this could not be determined from the data in this study. The
best-corrected visual acuity was statistically similar in all three IOL groups,
reflecting on average a six-line improvement over preoperative levels. The
uncorrected visual acuity of the modified prolate aspheric IOL was superior to
the other two groups. For the purposes of data collection and contrast testing
for this study, only vision with best correction was utilized.
The
data on functional acuity contrast testing in this study demonstrates that
cataract patients exhibit the lowest levels of contrast sensitivity of all the
groups tested in each of the four lighting levels. It can be expected when
testing patients in bright light (photopic) conditions, that pupil size will be
smaller and spherical aberrations induced by the lens, would be lessened. As
this study demonstrates, there is little difference between the preoperative
cataract, silicone and acrylic IOL groups in photopic conditions, with the
aspheric IOL group demonstrating a 38% to 47% increase in contrast values
especially at the highest spatial frequencies. The data for photopic testing
with glare for all three IOL groups demonstrate improved contrast testing when
compared to preoperative cataract levels.
The
contributions from reduced spherical aberration contributed by the aspheric IOL
should be maximized in mesopic conditions. The data clearly demonstrate that
under mesopic conditions, the aspheric IOL performs the best. The silicone IOL
group showed no increase in contrast sensitivity over preoperative cataract
levels. At the smallest target sizes of 12 to 18 cpd, where it is most difficult
for the patient to distinguish differences, the acrylic IOL group showed a 25%
to 50% increase over preoperative cataract levels, the aspheric IOL group 50% to
100%. This pattern was even more apparent when viewing the data for mesopic
testing with glare. There was no improvement in contrast testing with the
silicone IOL group. The acrylic IOL group demonstrated an increase of 36% over
preoperative cataract levels at 12 cpd and 50% at 18 cpd. The aspheric IOL group
demonstrated improved contrast testing at every frequency tested from 9% at 1.5 cpd, 23% at 3 and 6
cpd, 43% at 12 cpd to 100% at 18 cpd. The data speak for
themselves. The addition of an anterior, modified prolate optic to the IOL is
associated with up to 100% increase in contrast sensitivity levels and that this
improvement is demonstrable at all frequencies and most apparent at the highest
frequencies, in lowest light levels and lowest light levels with glare. Based
upon this data, one might expect this IOL to perform best for patients at night
and when there is a glare source, such as oncoming headlights from an
automobile.
What
is the relevance of functional acuity contrast testing to actual real life
visual demands and are the testing methods valid? Sine-wave gratings have been
used for over three decades in visual psychophysics11.
Using computer generated sine-wave gratings in a view-in tester, as was applied
in this study, eliminates the need to control room light levels, is accepted by
the patient, and allows the observer to control the light source and present the
target to one eye at a time. My previous work in contrast testing of bifocal and
multifocal IOLs (1989-unpublished observations-Kershner) utilizing the
Multivision Contrast Tester (MCT, Vistech Consultants, Dayton, OH USA) and in
this study, with the Optec series (Stereo Optical) have confirmed that these
instruments are an excellent standard by which test comparisons of contrast
thresholds and spatial frequencies can be made. Contrast sensitivity testing has
been shown to provide a consistently high degree of correlation with visual
performance22,25.
What
contribution can the lens material and optic size contribute to the potential
for improved contrast testing? This study compared three lens styles and two
materials, and two optic sizes. The
one-piece silicone IOL demonstrated the least improvement on contrast testing
and image contrast. The differences between this IOL and the aspheric IOL tested
is the silicone lens is a one-piece, plate haptic IOL. The RMX-3 silicone
material is similar to the polysiloxane silicone used in the aspheric IOL. It is
possible that any of these factors may contribute to the optical performance of
the IOL but in the case of the one-piece silicone IOL it is not likely to be the
material. The acrylic IOL by comparison, is a one-piece IOL made of a different
optic material, acrylate / methacrylate copolymer. It performed better than the
one-piece silicone IOL but not as good as the aspheric for uncorrected visual
acuity, image contrast and contrast sensitivity. It cannot be determined from
this study with certainty, whether the difference in IOL performance is due to
the material or the geometry of the optic. The optic sizes for the silicone and
acrylic IOLs were 5.5mm, the aspheric was 6.0mm. One could postulate that the
smaller optic sizes could be contributing to the loss of contrast sensititivity
at larger pupil sizes. In an elderly population, such as in this study, it is
uncommon to have naturally occurring pupil size greater than 5mm. That is one
reason manufacturers choose 5.5 or 6.0mm for optic sizes. Therefore, it would be
unlikely that optic size alone would contribute to the differences observed in
this study. Packer’s studies, compared an acrylic IOL with a 6.0mm optic to
the aspheric IOL tested in this study and failed to demonstrate a contributory
difference from optic size or material27. Another factor that could
be postulated to affect the performance of the IOLs tested is the lens capsule.
The contribution of the negative sphericity of the aspheric IOL versus the
positive spherical aberration contributed from the spherical IOLs should be the
primary factor in determining IOL performance with the testing parameters in
this study. Patients were screened for capsular opacification and if identified
and determined that it contributed to a decline in visual acuity from
postoperative levels, then the patient underwent YAG laser capsulotomy for that
eye prior to contrast testing. Only a small number of the one-piece silicone
IOLs (0.9%) required this treatment during the course of the study. Defocus from
incorrect IOL power or capsular opacification should not be significant
contributers to contrast testing performance11. Nonetheless, the
results of this study cannot rule out other factors also contributing to visual
performance of the IOLs.
Retinal imaging is an area where cataract surgeons have not
previously placed emphasis. With the early acceptance of IOLs, retinal surgeons
were concerned that visualization would be impaired for such procedures as
retinal detachment repairs or photocoagulation therapy. As cataract surgical
outcomes have improved, this concern is not commonly voiced today. In the
routine examination of the eye, ophthalmologists utilize an aspheric lens for
visualizing the retinal fundus in indirect ophthalmoscopy. This aspheric
handheld lens reduces spherical aberration allowing for clear undistorted
imaging out to the retinal periphery. Applying similar logic, one could
postulate that an aspheric lens implanted within the eye, may allow for better
clarity and image contrast when visualizing the retinal fundus. How best to test
this hypothesis? I used an innovative application of digital imaging to
determine if the image taken under controlled conditions of the retina by a
fundus camera, could be analyzed to determine if the image contrast was
different depending upon the IOL through which the photograph was taken. Are
these images subject to pupil size? In an effort to minimize pupil size as a
factor, all patients were photographed through a pupil size of 3-5mm. The
patients' own preoperative fundus photograph served as their control when
comparing the images to avoid the variable due to the pigmentation of the
patient's fundus. The threshold luminance maps represent the actual image data
for color and contrast of each fundus photograph. This data can be displayed
numerically. The difference from the lowest (lightest) values to the highest
(darkest) is the range of luminance. The
threshold luminance is represented by the lowest and the highest values.
Although the threshold luminance levels will vary from image to image, the
difference from lightest to darkest (contrast) can be measured and compared
between images and groups. Contrast is defined mathematically as the difference
between brightness of the light edge, subtracted from the dark edge, divided by
the sum of the two brightnesses. This numerical value can describe for us the
percent contrast of an image. Using these values, the study reveals that the
improvement in contrast of the retinal images were unchanged for the silicone
IOL group compared to preoperative cataract, improved only 3% for the acrylic
group but demonstrated 8% more contrast to the images obtained through the
aspheric IOLs for an improvement of 40% compared to preoperative cataract.
During
the training of fellows, I often use the expression, “what you see-in, is what
the patient sees-out”. If an
intraocular lens provides better contrast sensitivity testing with the patient
looking out, then this benefit should be apparent to the examiner when looking
in. How do we objectively assess the appearance of increased contrast to a
fundus image? The digital technology exists, although it is not generally
applied in this manner.
To my knowledge this study for the first time applies the technology
of digital photographic analysis to the image contrast of the retinal fundus.
This study demonstrates that retinal image contrast data for each IOL mirrors
the data obtained from completely different testing methods for functional
acuity contrast.
Wavefront
imaging has become the standard by which many refractive surgeons may soon be
imaging the refractive power of the human eye. By passing light into the eye
from a point source (the wavefront), the returned images can be recorded by
sensors outside of the eye. The deviations of these images or the point spread
function can be calculated and constitute the aberration or wavefront due to
irregularities in the refracting surfaces of the cornea, lens and fundus. The
modulation transfer function (MTF) describes the relationship between contrast
of an object and its image. Guiaro et al. demonstrated (using the MTF) that the
decline in visual performance in older individuals is due to changes in the
crystalline lens which interferes with the optical performance of the eye28.
Unlike wavefront analysis, which depends upon these refractive changes, contrast
image analysis is little affected by the focus of the image. In this study, the
only variable we have changed is the optical aberrations contributed by the
lens. The images displayed in figure 5 and the data in figure 6, represent
imaging we see-in and therefore represent the quality of the image the patient
sees-out. The preoperative cataract
retinal images showed the lowest difference in threshold luminance and the
lowest percent contrast of the four groups tested. The silicone and acrylic IOLs
showed a similar mean range in threshold luminance with no improvement in the
percent contrast for the silicone IOLs and only a fifteen percent increase in
contrast for the acrylic IOLs from the preoperative cataract group. By
comparison, the aspheric group had the greatest difference in threshold
luminance and the greatest percent contrast of the four groups tested exceeding
the preoperative cataract group by forty percent. The fundus retinal image
contrast analysis corroborates what was seen objectively in the patients with
functional contrast acuity testing. The anterior surface, modified prolate,
aspheric IOL (Tecnis Z-9000) is associated with more than a 100% increase in
functional acuity contrast and a four-fold increase in image contrast of the
patient's own retina imaging. The data demonstrates an even greater improvement
in the contrast testing for night vision.
The
theory behind an aspheric IOL is that it compensates for the average spherical
aberration of the human cornea in a cataract population and therefore should
offer superior optical performance when compared to a spherical IOL when testing
with pupil sizes of 5-6mm. At larger pupils sizes (as is seen in mesopic
testing) the degrading effects of any optical aberrations become more
significant25. Indeed the data reported for night vision and night
vision with glare is better for this IOL. Packer et al. performed a prospective
randomized trial of the aspheric lens and found that the Tecnis Z-9000 IOL
provided significantly better contrast sensitivity at 1.5 and 3 cpd under
mesopic conditions and at 6, 12, and 18 cpd under photopic conditions28.
Their group further compared the aspheric IOL with an acrylic IOL, the AR40e IOL (Opti-EdgeTM, Advanced Medical Optics, Santa Ana, CA, USA). Their
results demonstrated that at peak contrast sensitivity, the Tecnis IOL group had
38.5% greater contrast under photopic conditions and 77.9% greater under mesopic
conditions28.
Why
does this lens work the way it does? It is now well established that the
contribution from the cornea (primarily it’s anterior surface) and the
crystalline lens, each play a compensatory role in neutralizing the spherical
aberration within the human eye29-30. The designers of the anterior
modified prolate aspheric IOL, estimated the total ocular spherical aberration
from a pool of patients and the rotationally symmetric aberration contributed
from the IOL to create an IOL, that in most patients, would neutralize or reduce
the average spherical contribution from the cornea25. The positive
spherical aberration of the human cornea (approximately one-half of a sphere or
–0.52) may compensate for the negative spherical aberration of the human
crystalline lens seen in youth6. The positive spherical aberration of
the cataract in the aging eye may add to this aberration and make the quality of
vision worse in elderly patients. Replacing the crystalline lens with a
spherical IOL that has an inherent positive spherical aberration may fail to
correct the error created by the cataract. The theory behind the development of
an aspheric optic IOL is based upon research that an IOL that mimics the
negative spherical aberration of the youthful lens, may reduce the ocular
aberration of the aging eye and therefore improve the optical quality of the
pseudophakic eye25.
Our
patients today benefit from our success with modern cataract surgery. Today’s
procedures are quicker, safer and provide better uncorrected visual acuity than
ever before possible with surgery. Along with these improved outcomes however,
comes the burden that surgeons must continue to strive for better functional
visual performance for our patients with the least disability from our
procedures. At one time, simply removing the cataract was sufficient, banishing
the patient to a lifetime of visual disability with aphakic spectacles. More
modern approaches to the removal of cataracts have allowed millions of people
worldwide to see better with spectacles and an intraocular lens. With today’s
high technology IOLs and microminiaturized surgical techniques, many people are
now spectacle free following cataract surgery for most tasks1. In the
not too distant future, an IOL that restores accommodation and eliminates the
need for reading glasses may become available. Improving visual acuity for all
distances of visual need may just not be enough. We as ophthalmic surgeons,
entrusted by our patients with their most precious gift of vision, must strive
to provide a better quality of vision. Improved contrast sensitivity may be just
what the patient needs. The ability of our patients to perceive images under a
variety of different lighting conditions, maximizing their perception of image
contrast, especially at night, may bring us closer to the ultimate goal of super
vision-better visual performance than has ever before been possible.
The ability to improve contrast sensitivity in our cataract patients is
no longer just a theory, it is today a scientifically, demonstrable reality. A
new standard of care awaits us as cataract surgeons, we must now rise to meet
the challenge.
Conclusions
This
prospective, randomized study compares an aspheric IOL with conventional
spherical silicone and acrylic lenses on retinal image contrast and functional
visual performance. The results demonstrate that postoperative uncorrected
visual acuity is improved in all three IOLs tested with the greatest improvement
in the aspheric group. An improvement in functional acuity contrast testing
cannot be expected with cataract extraction and IOL implantation alone. Patients
implanted with the one-piece silicone IOL failed to demonstrate improved
contrast testing even though there was an improvement in visual acuity. The
one-piece acrylic IOL demonstrated improved contrast testing under some lighting
levels when compared to preoperative cataract, but the increased values were
significantly less than that seen with the aspheric IOLs. Although the clarity
of the retinal imaging was improved in all groups over the preoperative cataract
images, the retinal image contrast with the silicone and acrylic IOLs was
virtually the same as that measured for the preoperative cataract images.
Bibliography
1.
Kershner, RM. Clear Corneal Cataract Surgery and the Correction of Myopia,
Hyperopia and Astigmatism. Ophthalmology 1997:104(3):381-389
2.
Mehta KR, Sathe SN, Karyekar SD. The new soft intraocular lens implant. J Am
Intraocul Implant Soc 1978;4:200-5
3.
El-Maghraby A, Kraff MC, Raanan MG. Foldable IOL technology:an overview. In:
Martin RG, Gills JP, Sanders DR. Foldable intraocular lenses. Thorofare, NJ:
SLACK,1993;chap. 1
4.
Kershner, RM. Toric Lenses for Correcting Astigmatism in 130 Eyes. Discussion,
Ophthalmology, 2000;107:1776-82
14.
Kershner RM. Cataract surgery technique using topical anesthesia. In: Fine IH,
Fichman RA, Grabow HB., Clear-corneal cataract surgery and topical anesthesia. Thorofare, NJ:SLACK,1993;141-53.
15. Kershner RM. Corneal anatomy and the no-touch
technique. In: Fine IH, Fichman RA, Grabow HB. Clear-corneal cataract surgery
and topical anesthesia. Thorofare, NJ:SLACK, 1993;79-84.
16.
Kershner RM. Embryology, anatomy and needle capsulotomy. In: Koch PS,
Davison JA, eds. Textbook of advanced phacoemulsification techniques.
Thorofare, NJ:SLACK,1991; 35-48.
17.
Kershner RM. One-step forceps for capsulorhexis. J Cataract Refract Surg
1990;16:762-5.
18.
Fine IH. Cortical cleaving
hydrodissection. J Cataract Refract Surg 1992;18:508-12.
24.
Nio Y, Jansonius N, Fidler, V, Geraghty, E, Norrby, S, Kooijman, A.
Age-related changes of defocus-specific contrast sensitivity in healthy
subjects. Ophthal Physiol Opt. 2000;20:323-334
©2003. Robert M. Kershner, M.D., F.A.C.S. All rights reserved.
Robert M. Kershner, MD, FACS-Eye Laser Center-Suite 303-1925 West Orange Grove Road-Tucson, AZ 85704-1152 Phone 520-797-2020, Fax 520-797-2235 e-mail: Kershner@EyeLaserCenter.com
For more information, visit: http://www.EyeLaserCenter.com/PhysicianResource.htm